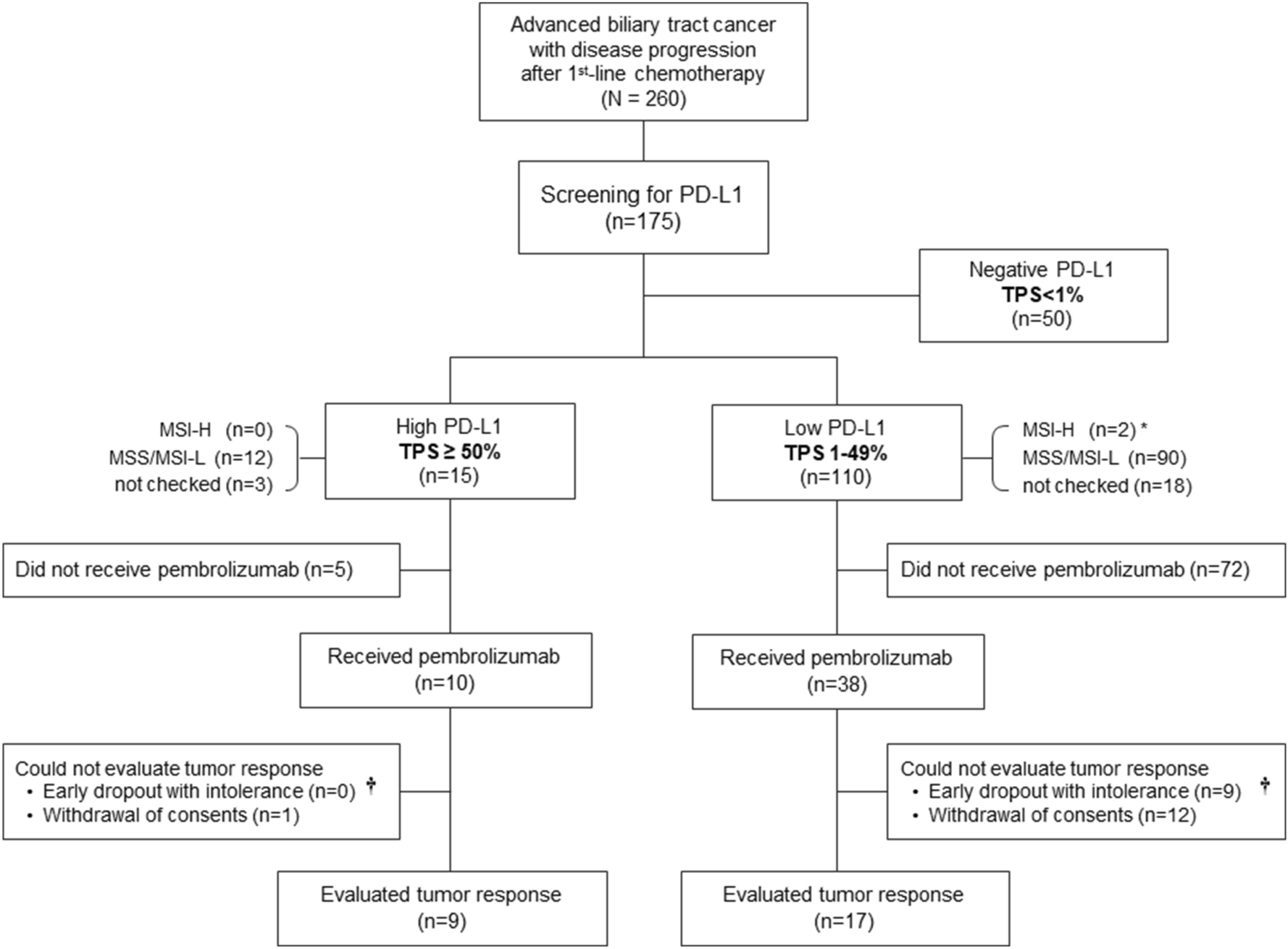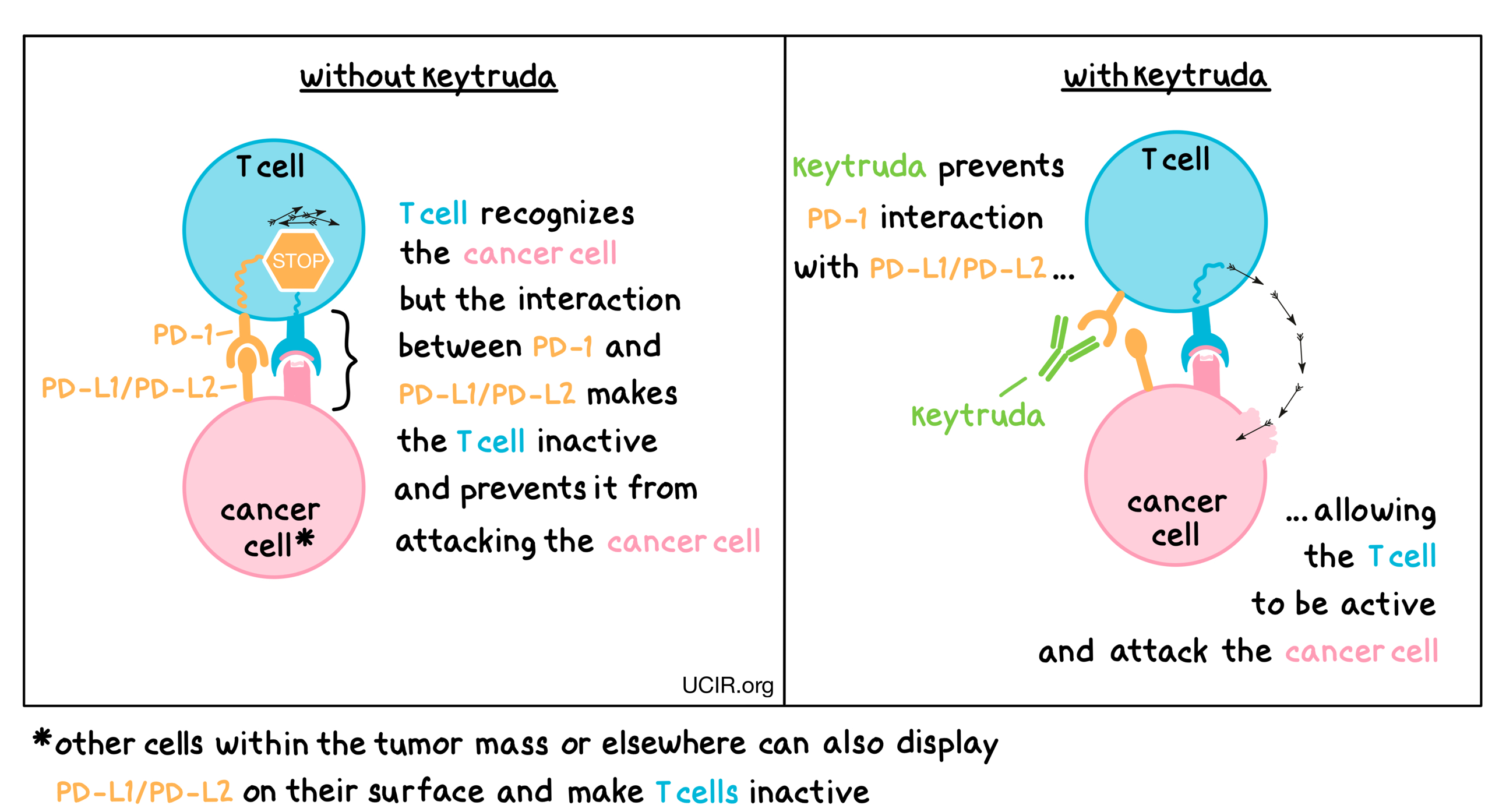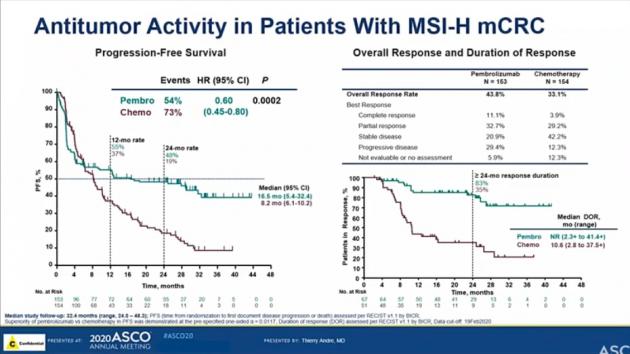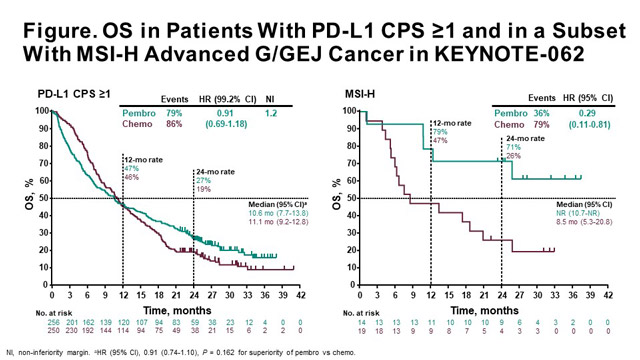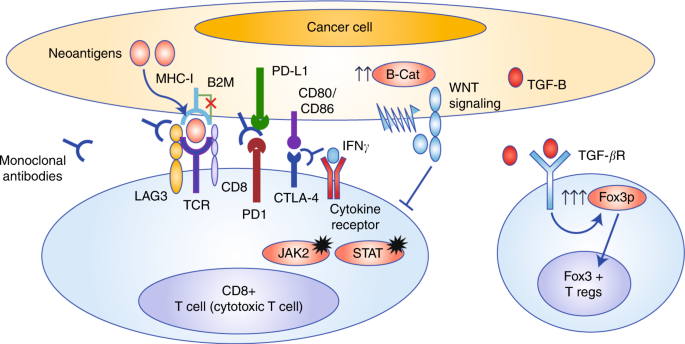
Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms | British Journal of Cancer

FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors | Semantic Scholar
Approval of pembrolizumab (MSI-H/dMMR) and considerations for site-agnostic development of drugs in oncology
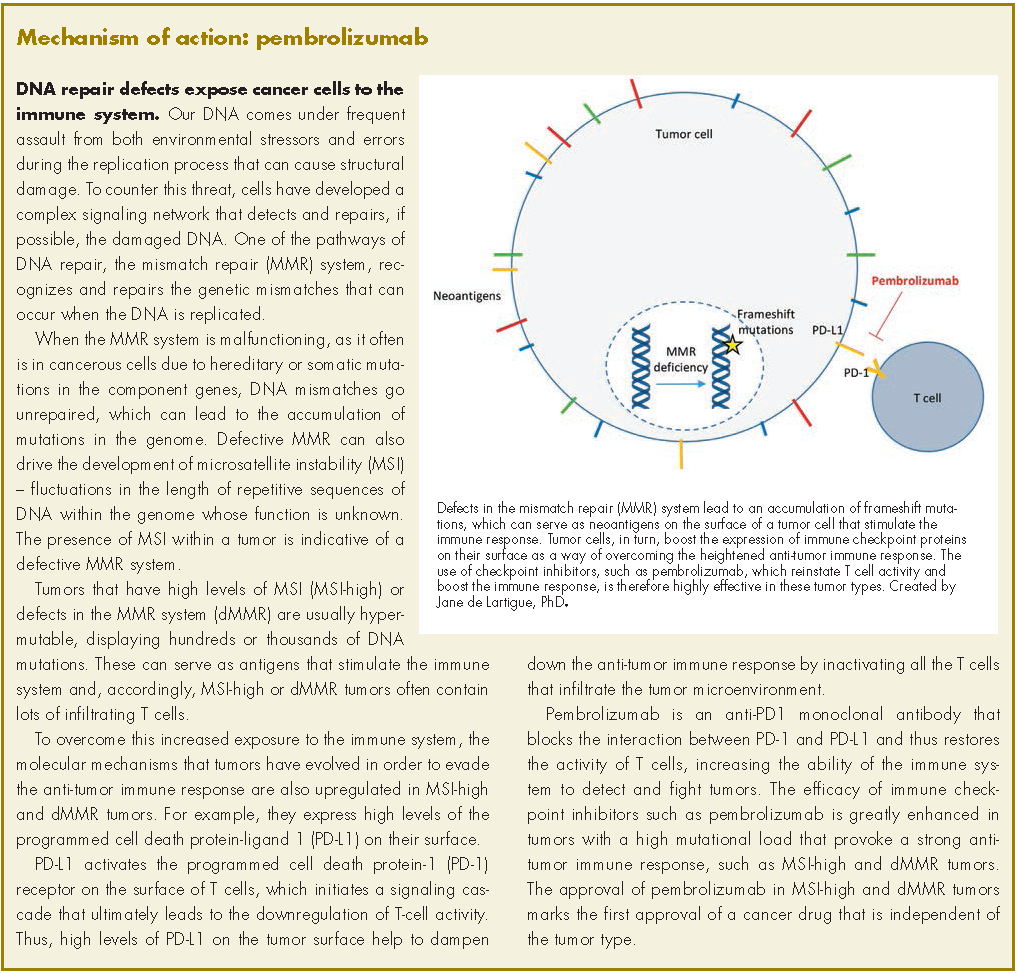
Pembrolizumab for dMMR/MSI-H tumors marks first tumor agnostic FDA approval | MDedge Hematology and Oncology

Best PSA response on pembrolizumab in men with MMRd/MSI-H PC. Best %... | Download Scientific Diagram

FDA Converts to Full Approval Indication for KEYTRUDA® (pembrolizumab) for Certain Adult and Pediatric Patients With Advanced Microsatellite Instability-High (MSI-H) or Mismatch Repair Deficient (dMMR) Solid Tumors | Business Wire

Study of Pembrolizumab (MK-3475) vs Standard Therapy in Participants With Microsatellite Instability-High (MSI-H)

ESMO 2022: Pembrolizumab in microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) advanced solid tumors: An update of the phase II KEYNOTE-158 trial

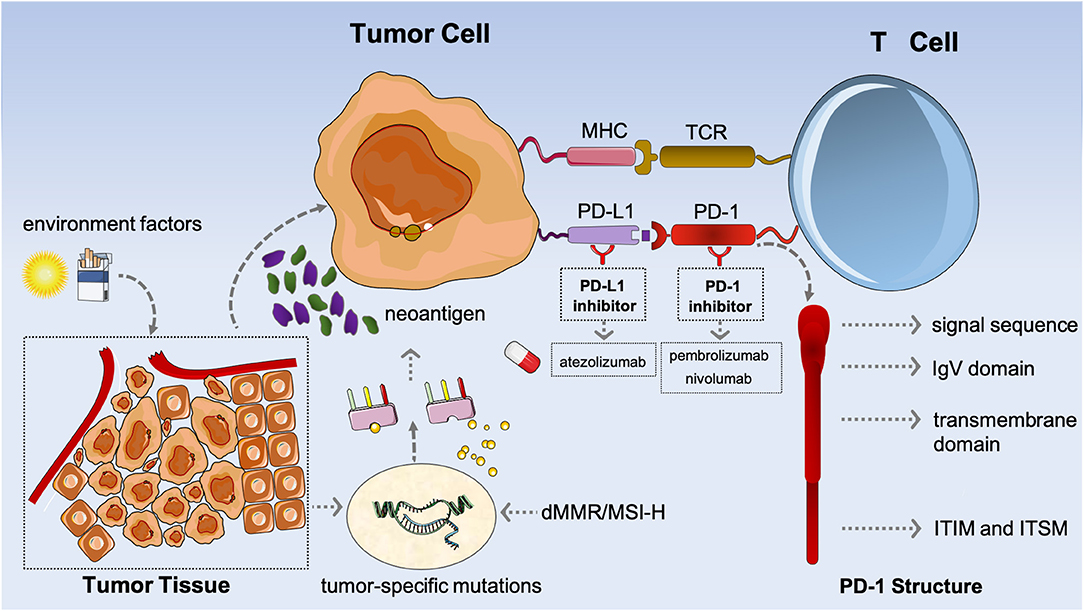
Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers | SpringerLink